1/26/22 Steering Committee Meeting
We had a great first steering committee meeting of the year with about 35 members joining and participating.
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
Watch the recording of the meeting above.
0:00-3:46 Introduction
3:47-12:33 CLIA Waiver Project
12:34-14:55 FDA Transition Plan
14:56-24:15 Noteworthy Papers
24:16-30:05 Truthing & Validation Update
30:06-40:18 PANDA challenge discussion
40:19-46:50 Patient Advocacy Update
46:51-47:22 ISPOR
47:29-49:32 ONC Technical Specifications
49:33-52:23 FDA Updates
52:24-53:20 MDIC
53:21-55:14 Upcoming Events
55:15-end Discussion and concluding remarks
Meeting Summary
“CLIA Waiver” for Digital Pathology
Learn more on the project page here
Need input and/or supplementation from Steering Committee on next steps
what do folks think about attaining additional regulatory input from CLIA State Survey Agency Contacts?
send out a survey monkey to get a larger sample of responses
hear whether they would provide input themselves on how they would provide participate
FDA Transition Plan
FDA Website link to comment here
Posted in Federal Register as notice, currently provided for commenting
2 elements of transition plan:
medical devices with issued EUA towards a more permanent solution
and/or medical devices that fall within enforcement policies
Marble et al. paper describes changes during pandemic implemented early 2020 are still in effect
Noteworthy Papers
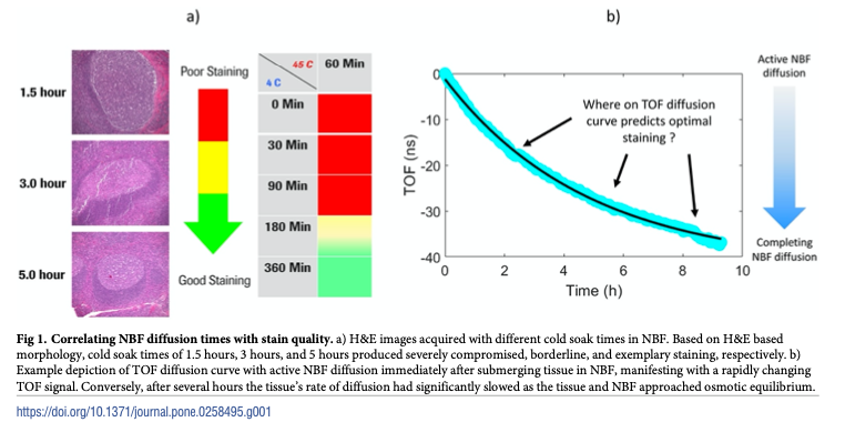
Bauer et al. Making a science out of preanalytics: an analytical method to determine optimal tissue fixation in real-time (link)
Morjaria et al. Strategic thinking in test selection for mass SARS-CoV-2 testing (link)
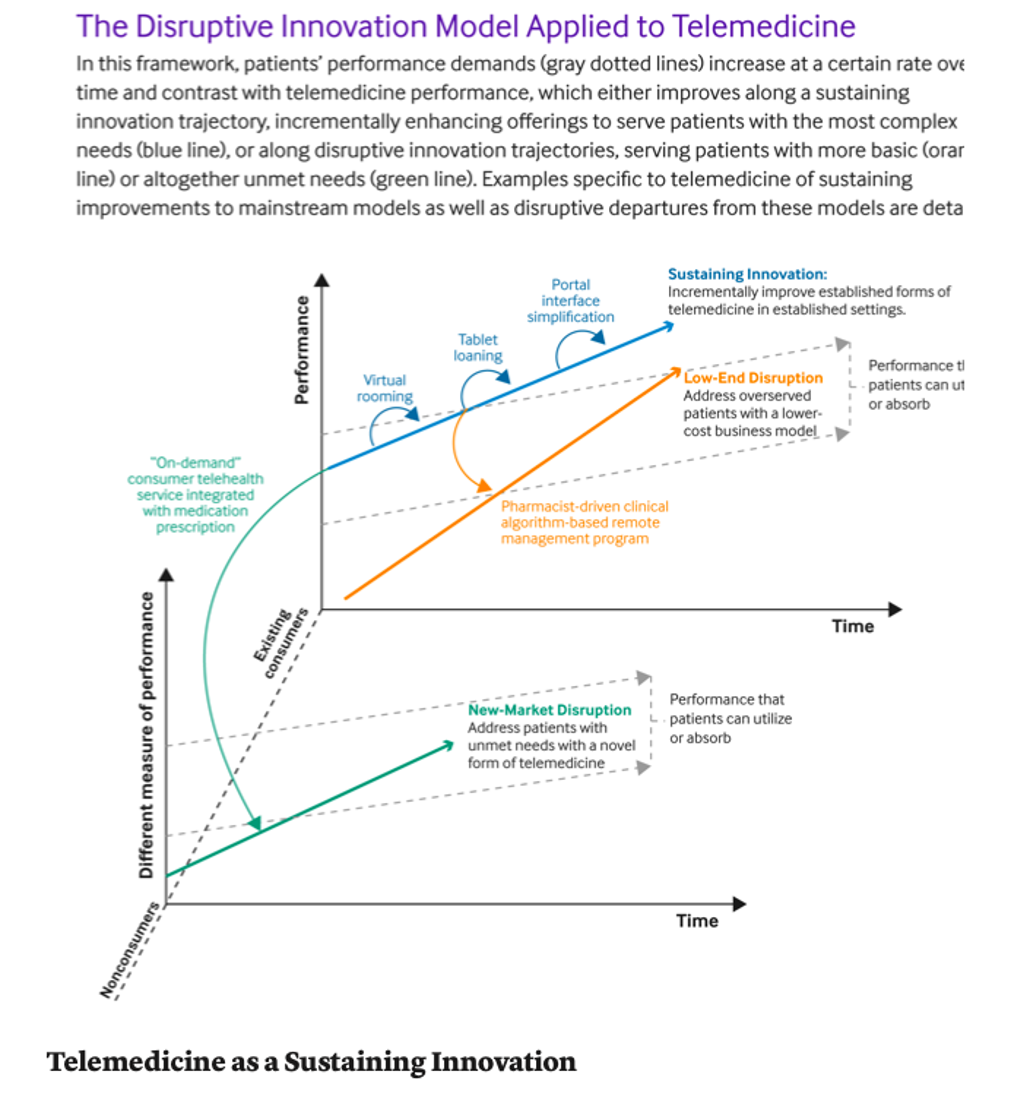
Lee et al. Disruptive and sustaining innovation in telemedicine: a strategic roadmap (link)
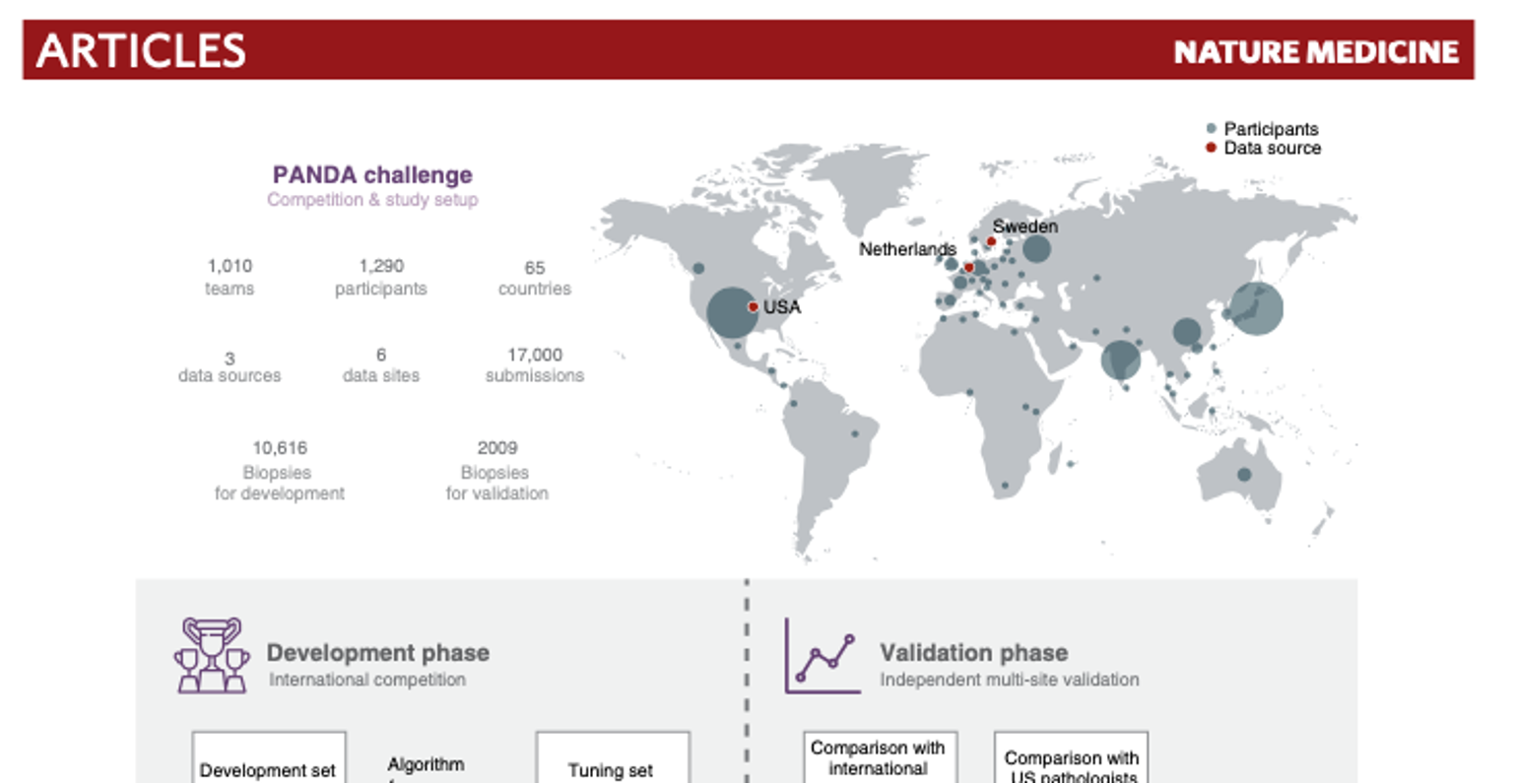
Bulten et al. Artificial intelligence for diagnosis and Gleason grading of prostate cancer; the PANDA challenge (link)
Discussion:
Field is currently lacking studies of algorithm in the hands of pathologists (not algorithm vs truth but the algorithm in the hand of the end user)
Augmented AI paper example (link)
Language needed to clarify (technical vs clinical validation, read studies, autonomous vs aid, etc.)
Link to PIcc glossary. Feedback, comments and additions are welcome
Patient Advocacy Update
Sepsis Alliance
Sepsis = multi-billion dollar impact on healthcare industry
cross collaborative community effort, with introduction by Michelle Tarver, FDA
first meeting happened on 1/19/22
discussing how to become a collaborative community
APPIA
focusing on a pre-analytics project: TOPS program administered through partnership with NSH (link)
presenting scope and materials of PIcc at their upcoming board meeting
Additional meetings pending with other patient advocacy groups
If you have a contact, please share
HIT (ONC) Technical specifications
Complexity of standards
Project US@ (link)
FDA Updates
Clinical Investigator Training Course Update (link)
RWE slides (link)
2022 Summer OSEL Regulatory Research Experience (SORRE) (link)
Initiative for real world data collection (link)
PIcc Updates
Download Truthing & Validation group here
UPDATE based on questions/discussion during the meeting:
PIcc has a glossary of introductory overview of specific terms used in the context of regulatory science and digital pathology.
Brandon Gallas (FDA) created a wiki page to help image-based AI/ML developers and industry links pointing to related FDA guidance documents, links to first-of-a-kind devices, and presentations from FDA staff.
Brandon provided an observation that many or most of the studies on AI/ML for digital pathology images report analytical performance and that clinical performance is understudied. Brandon clarified that analytical performance is calculated by comparing the model outputs to a reference standard, often referred to as standalone performance (especially in radiology). Brandon also clarified that clinical performance is calculated by comparing the performance of pathologists with and without the AI/ML model outputs; each interpretation mode is compared to a reference standard. Such a clinical performance study is sometimes referred to as a reader study. You can refer to these (radiology) guidance documents for more information.
Computer aided detection (CADe) guidance – Radiology
TILS survey being finalized
website domain updating to pathologyinnovationcc.org
MDIC Updates
Job positions available (link)
Emerging Healthtech Series (link)
MDICx: AI/ML Framework Public Comment Q&A (link)
Upcoming Events
2/22/22 10AM-1PM ET PMLS Virtual Series 2022 – Precision Pathology (link)
2/22/22 12-1PM ET FOCR Virtual Meeting: Supporting Development of Diagnostic Tests for Unmet Needs (link)
Discussion