2/23/22 Steering Committee Meeting
This month’s meeting was well attended by a diverse group of stakeholders.
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
Watch the recording of the meeting above.
0:00-3:32 Introduction
3:33-4:44 FDA QMS harmonization plan
4:45-7:15 DIDSR Opportunities
7:16-7:59 Transition Guidance for COVID-19 Devices
8:00-8:30 CDRH Annual Report
8:31-10:17 No Surprise Act
10:18-16:21 Privacy Project Updates
16:22-18:04 Closing Gaps in Cancer Screening
18:05-23:26 Patient Advocacy
23:27-32:58 Featured Papers
32:59-40:12 Upcoming Events
40:13-end Discussion
Meeting Summary
FDA QMS Harmonization Plan
Harmonizing and Modernizing Regulation of Medical Device Quality Systems
FDA Transition to ISO 13485:2016 (download)
DIDSR Opportunities
expanding fellowship opportunities (for PhD and masters candidates) to include professors with the aim to get higher level collaboration
FDA Webinar on Draft Guidances on Transition Plans for COVID-19 Related Medical Devices
No Surprise Act
related to a few PIcc projects underway
signed into law as part of the Consolidated Appropriations Act of 2021
AMA toolkit (link to download)
Privacy Project Updates
ISPOR abstract not accepted
Health Data Use and Privacy Commission Act (link to legislation)
CommonWell Health Alliance (link)
Closing Gaps in Cancer Screening
Report to the President from the President’s Cancer Council (link)
independent of the Cancer Moonshot program
Patient advocacy updates
In contact with Sepsis Alliance to help them get started as a collaborative community
FOCR whitepaper: Expedited Development of Diagnostics for Therapies Targeting Rare Biomarkers or Indications (link)
APPIA board presentation
join pre-analytics planning meeting on Wednesday, March 2, 2022 at 1:00-2:00PM EST
Featured papers
Bulten et al. Artificial intelligence assistance significantly improves Gleason grading of prostate biopsies by pathologists (link)
Nguyen et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients (link)
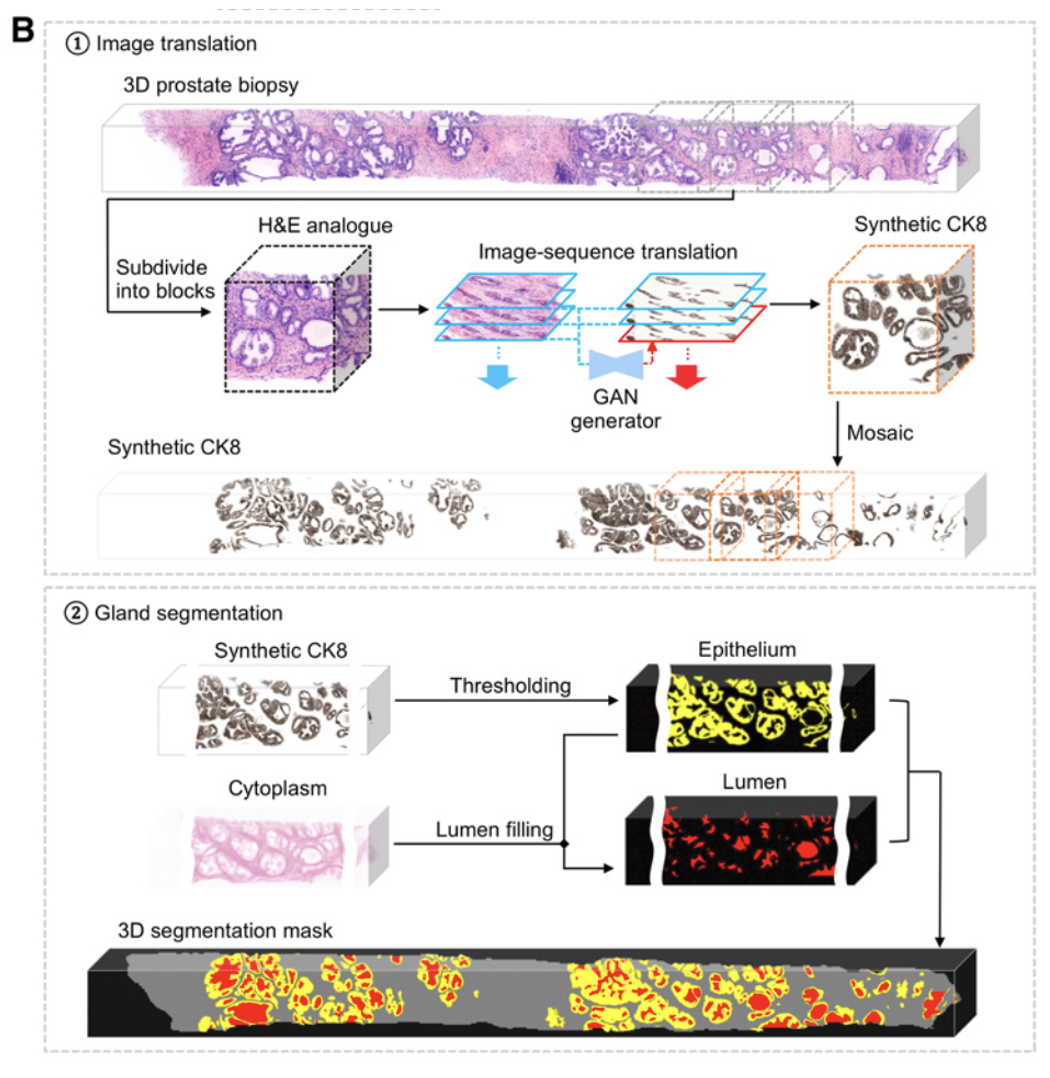
Xie et al. Prostate Cancer Risk Stratification via Nondestructive 3D Pathology with Deep Learning–Assisted Gland Analysis (link)
Reid et al. Physician Compensation Arrangements and Financial Performance Incentives in US Health Systems (link)
von Stillfried et al. First report from the German COVID-19 autopsy registry (link)
Smit et al. Quality control of whole-slide images through multi-class semantic segmentation of artifacts (link)
Gallas et al. FDA fosters innovative approaches in research, resources and collaboration
Upcoming Events
March 1, 2022 at 1:00-2:30PM EST FDA Webinar - Principles for Selecting, Developing, Modifying, and Adapting Patient-Reported Outcome Instruments for Use in Medical Device Evaluation - Final Guidance (link)
March 19, 2022 at 9:00PM PST Informal PIcc meeting at USCAP (link)
March 30, 2022 at 3:00-4:00 PM EST Next PIcc steering committee meeting (link)