11/30/22 Steering Committee Meeting
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
0:00-2:44 Introduction
2:45-16:55 FDA Overview
16:56-19:09 WHO/IARC Updates
19:10-25:52 Legislative Updates
25:53-29:12 MDIC Updates
29:13-30:56 Professional Societies
30:57-34:58 Project Updates
34:59-37:01 Ethics, Diversity & Inclusion
37:02-40:51 Patient Advocacy
40:52-58:23 Resources
58:24-59:00 Payor
59:01- Events & closing
Watch the recording of the meeting above.
Meeting Summary & Content Links
FDA Overview
Spotlight: Digital Health Regulatory Science Research Opportunities
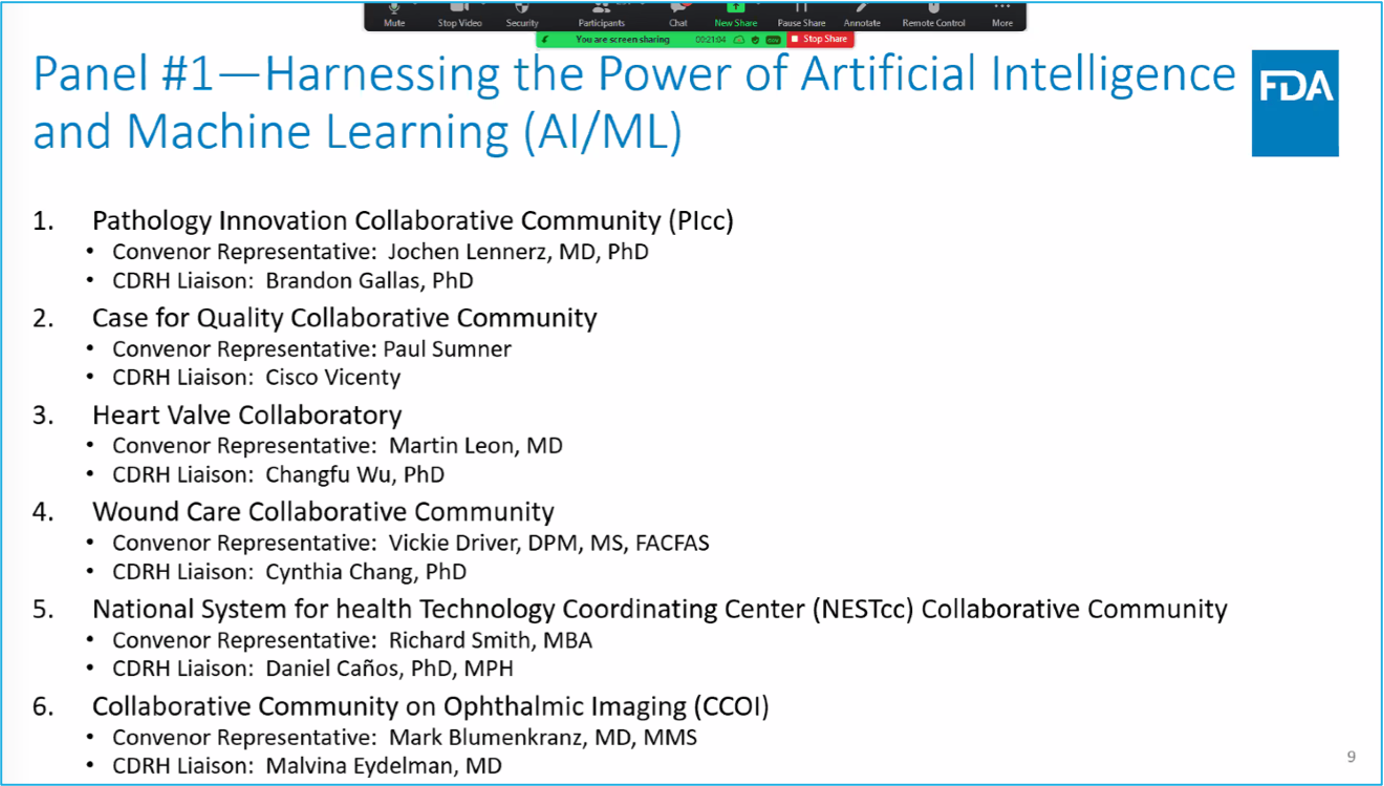
mentions digital pathology and collaborative communities
Collaborative Community Townhall on November 10, 2022
presentation by PIcc
FDA internal town hall meeting
Modeling & Simulation approach (download PDF)
MDDT Program (link)
a few new designations across different subcategories
example: CHRIS color additives
Cybersecurity - November 15 update (link)
possibility for a draft guidance coming soon?
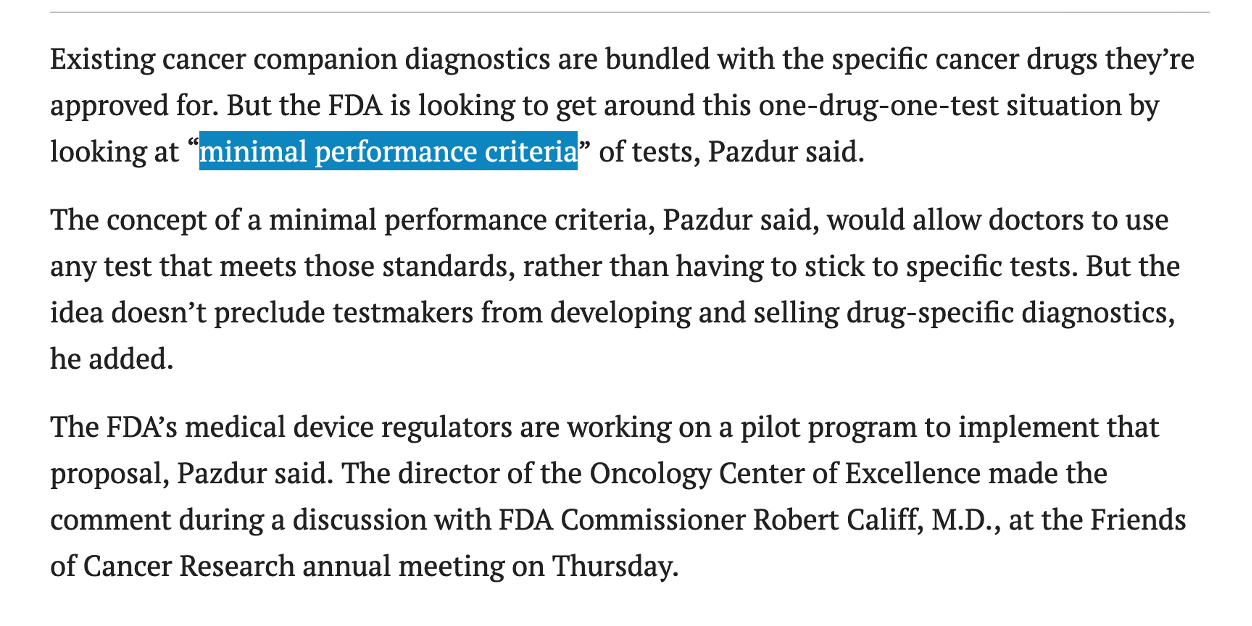
November 18 news: official statement by Pazdur for FDA to look into an extension of companion diagnostic program (link)
DHCoE Digital Health Center of Excellence (link to overview)
HTT Project Updates
trying to get pivotal study off the ground, aiming for beginning of new year
goal of project: provide methodology and dataset to validate AI algorithms
installing the WSI viewer caMicroscope on precision FDA for data collection
December 5-7, 2022 FDA Digital Transformation Symposium (link)
Real World Evidence Program (download)
illustration and outline in NEJM (download)
WHO/IARC Updates
Understanding the use of evidence in the WHO Classification of Tumours: a protocol for an evidence gap map of the classification of tumours of the lung (link)
Medicine should be considered a human right - WHO Model lists of essential medicines
working on extension of list to include essential diagnostics (link)
IARC Perspective on Oral Cancer Prevention (link)
Legislative Updates
Summary by Health IT Security - White House sets standards for healthcare cybersecurity (link)
VALID Act: the story continues…. (link)
might be attached to end of year spending bill
December 5 - Educational session for congress personalized medicine caucus (link)
Better lab tests can ensure precision medicine is truly precise (link)
recent annual report to the nation on the status of cancer (link)
What’s the difference between device and technology? (download guidance document)
Switzerland has decided to accept medical devices with FDA approval for the welfare of their own population (link)
Voluntary Alternative Pathway: Agile Regulation - system where you voluntarily submit performance updates/meaningful data creating an ongoing communication in terms of regulatory oversight (link)
MDIC Updates
New Approach to Clinical Trial Design Helps Medical Devices Better Meet Patient Needs and Priorities (link) (link)
Cybersecurity Threat-modeling Virtual Bootcamps: 12/12-12/16, 2022 and 3/13-3/17, 2023 (link)
Medical Device Cybersecurity Maturity: MDIC Industry Benchmarking Report 2022 has been released (link)
Coming Soon:
AI/ML in IVDs: Framework for a Predetermined Change Control Plan (PCCP) for AI/ML-Enabled IVDs, including both Software as a Medical Device (SaMD) and Software in a Medical Device (SiMD)
5G-enabled Healthcare Technologies: MDIC Landscape Report (Coming December 2022)
Computational Modeling & Simulation (CM&S) in Medical Device & Diagnostics: Case Studies and Landscape Analysis (Coming December 2022)
Please contact Noor Falah nfalah@mdic.org or Jithesh Veetil jveetil@mdic.org with any questions about MDIC initiatives
Professional Societies
AMP proposal for moderinization of CLIA regulations (download)
new CAP council - Council on Informatics and Pathology Innovations (link)
CAP received contract with FDA (link to press release)
DPA is currently planning next meeting and materials from Path Visions are now availble
Project updates
ctDNA
Liquid Biopsy for Precision Onology Summit February 14-16 (link)
Ruhen et al. Molecular Characterization of Circulating Tumor DNA in Pediatric Rhabdomyosarcoma: A Feasibility Study (pubmed link)
AI Grand Challenges
initial results from TIGER challenge were released (link)
CPIM
Special Issue: Proceedings of the STP 41st Annual Symposium: Toxicologic Pathology of the Hematopoietic System (link)
Ethics, Diversity & Inclusion
Nikolaos M. Siafakas. Do we need a Hippocratic Oath for artificial intelligence scientists? (download)
Carruthers et al. Representational ethical model calibration (download)
Miller et al. the Development of Children’s Gender-Science Stereotypes: A meta-analysis of 5 Decades of U.S. Draw-a-Scientist Studies (download)
Patient Advocacy
November 17 - FOCR annual meeting
HRD Harmonization Project data presented (download)
APPIA general tissue handling guidelines (link)
bioRxiv - A personal, reference quality fully annotated genome from a Saudi individual (link)
Resources
ML Tools for Pathology Images (link)
high-five to Heather from PixelScientia.com
Free No-Code NLP Platform (link)
Regulatory Aspects on Digital Pathology - Joe Sirintrapun seeking co-authors, soliciting volunteers. Reach out if you’d like to join him.
Book Title: Digital Pathology: Implementation in Clinical Practice: AI applications within Digital Pathology Framework
Co-Editors: Meera Hameed and Matthew Hanna
Publisher: Elsevier
Modesitt et al. How to write like a pro and publish like a boss (download)
Ecarnot et al. Writing a scientific article: A step-by-step guide for beginners (download)
Report from Deloitte: The future of diagnostics Technology driven personalised and preventative healthcare in Europe (link) (download)
Marine, Dawson & Dawson. Non-genetic mechanisms of therapeutic resistance in cancer (download)
Lomakin et al. Spatial genomics maps the structure, nature and evolution of cancer clones (link)
Lennerz et al. A unifying force for the realization of medical AI (download)
Huang et al. The landscape of mRANCE nanomedicine (download)
Bidgoli et al. Bias reduction in representation of histopathology images using deep feature selection (download)
Brynolfsson et al. Haralick texture features from apparent diffusion coefficient (ADC) MRI images depend on imaging and pre-processing parameters (download)
Zhou et al. Multi-Site Cross-Organ Calibrate Deep Learning (link)
Yamaguchi et al. Trastuzumab Deruxtecan in Anti–Human Epidermal Growth Factor Receptor 2 Treatment– Naive Patients With Human Epidermal Growth Factor Receptor 2–Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial (download)
Wan et al. Prediction of early-stage melanoma recurrence using clinical and histopathologic features (download)
Carobene et al. Where is laboratory medicine headed in the next decade? (download)
Jansen et al. The vendor-agnostic EMPAIA platform for integrating AI applications into digital pathology infrastructures (download)
Austin. Opportunities and challenges in translational science (download)
Kammula et al. Characterization of oncology clinical trials using germline genetic data (download)
Ledford. Closing in on cancer’s deadliest mutations (download)
Jorensen & Westergaard. Predicitive biomarkers abd personalized pharmacotherapy (download)
Sadik et al. Impact of clinical practice gaps on the implementation of personalized medicine in advanced NSCLC (download)
Wahid et al. The coming decade in precision oncology: six riddles (link)
Lami et al. Overcoming the interobserver variability in lung adenocarcinoma subtyping (download)
Kline et al. Multimodal machine learning in precision health: a scoping review (download)
Lyubetskaya et al. Assessment of spatial transcriptomics for oncology discovery (download)
Golden et al. Emerging approached to redressing multi-level reproductive health disparities (download)
Damrauer et al. Collaborative study from the Bladder Cancer Advocacy Network for the genomic analysis of metastatic urothelial cancer (download)
Kather et al. Medical domain knowledge in domain-agnostic generative AI (download)
Jiang et al. A quantitative proteome map of the human body (download)
Payor Updates
Abramoff et al. A reimbursement framework for AI in healthcare (download)
Parikh & Helmchen. Paying for AI in medicine (download)
Upcoming events
Final meeting of the year will take place on Wednesday, 12/21 based on results from in-meeting poll
December 2 Coming Reimbursement Opportunities for Digital Pathology (link)
December 8 FDA Forecast: What’s Next for the FDA in 2023 (link)
December 5-7 FDA Digital Transformation Symposium (link)
December 7 Public Workshop - Appropriate Use of Consensus Standards (link)
December 7-8 FDA Clinical Investigator Training Course (link)
Next in-person PIcc meeting in planning stages - March 4-5 OR March 18-19